[ZenithT] is expanding globally with its advanced medical devices.
- Name 관리자
- Date 2023-11-21
ZenithT is expanding globally with its advanced medical devices.

Q. Please provide an introduction about (ZenithT Co., Ltd)
A. Our company was founded in 2016 and achieved mid-tier industry sales within five years of obtaining GMP certification for medical device manufacturing and quality management in 2018. This achievement is even more significant as it was accomplished solely through domestic sales without overseas exports.
Currently, our company is actively expanding export channels to international markets such as Europe, Southeast Asia, and the United States through domestic and Gyeonggi Province export support programs. We are participating in such exhibitions as EUROSPINE, K-MED EXPO, Hospital Expo 2023, etc. to increase our overseas market presence and focus on product development based on customer feedback.

Minimally Invasive Device for Spinal Surgery
Q. Please describe the features and advantages of the single-use minimally invasive spine surgery system.
A.The trend is shifting towards replacing conventional surgical methods with minimally invasive procedures, which offer advantages such as reduced patient burden and shorter recovery times.
Traditional surgical methods, which involve fixation of the vertebrae and intervertebral discs or direct removal of the affected area, require incisions and pose significant burdens on patients, along with extended recovery times. To overcome the drawbacks of conventional surgeries, our company has developed a medical device that utilizes minimally invasive techniques. This device utilizes the epidural space between the spine and nerves to expand, remove, and deliver medications to the target area, reducing patient burden and facilitating quicker recovery.
All devices inserted into the human body are made from biocompatible materials of the Poly series, ensuring compatibility with the human body, and they are designed for single-use to prevent cross-contamination.
ZenithT's minimally invasive medical devices allow for same-day admission and discharge of patients, significantly reducing recovery time and lowering patient medical costs. These devices are gaining recognition in the market for their benefits.
Q. What are the plans for entering the international market and the future business direction or blueprint?
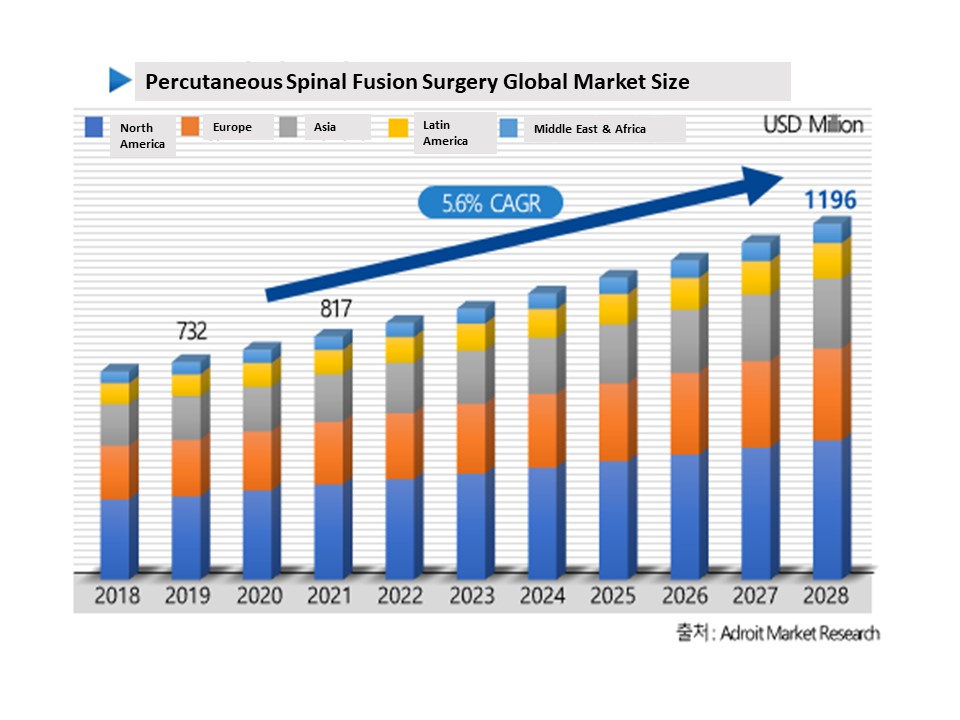
A. With the global aging population on the rise, the spinal medical device market has been growing at a rate of 5.6% annually. As of 2023, the market size for minimally invasive spinal surgery medical devices is approximately KRW 1.5 trillion.
Our company is currently in the process of obtaining ISO 13485 certification for overseas exports and plans to expand exports to Southeast Asia, Europe, and the United States through CE Marking in 2024 and FDA certification in 2025. To facilitate this expansion, we are actively participating in various international exhibitions to promote our products.
Website:https://www.zenitht.kr/?lang=en