[ILOODA] Competing in the Field of the Three Principles of Aesthetic Devices
- Name 관리자
- Date 2022-10-20
Competing in the Field of the Three Principles of Aesthetic Devices

Q. Please introduce ILOODA to us.
Specialized development in medical devices and product planning capabilities
A. ILOODA is a company that specializes in energy-based aesthetic devices using laser and high-frequency energy used by dermatologists and plastic surgeons. ILOODA was founded in 2006, and listed on KOSDAQ in August 2020. All of our products are medical devices approved by the Ministry of Food and Drug Safety. From the early stages of planning to device development, healthcare experts are involved in the entire process to develop devices that are fit for the market. This is our proprietary development process which sets our product capabilities apart from those of other companies. In 2019, our company was designated as a Global Hidden Champion by the Ministry of SMEs and Startups, and in 2020, we were designated as an innovative medical device company by the Ministry of Health and Welfare. In line with the changing environment of the plastic surgery and beauty market, we will continue to focus on technology innovations and develop new devices to reach KRW 50 billion in sales by 2023. By expanding our field of business to the digital healthcare and at-home care market, we hope to grow to become a globally renowned company.
Q. Briefly explain what the “energy-based aesthetic device” is.
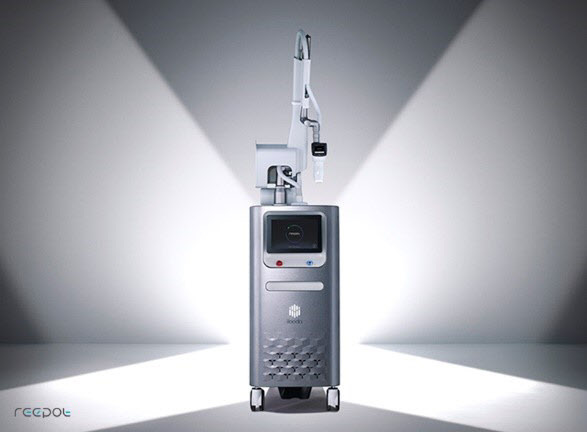
Launch of the next-generation laser device, Reepot
A. Our laser products are general-purpose laser surgical devices that can treat skin diseases using solid-state laser (Ng-yag source) and diode laser. Our recently launched Reepot is a medical device that uses vasculature salvage laser surgery (VSLS) technology and a laser with a wavelength of 532 nm. This combination protects the skin during the procedure by helping the skin maintain a certain cooling temperature while simultaneously delivering strong energy. Furthermore, the device also enhances treatment efficiency by identifying the accurate location of the area that needs treatment and selectively shooting energy in these target areas. Another type of general-purpose surgical device that uses high-frequency waves is our signature device, which is part of our line of Secret devices. Tiny needles penetrate the skin to transmit high-frequency currents to improve the skin. We also have Acutron, an electric surgical device that is optimized for microtissue operations such as delicate procedures in ophthalmology, and CURE.J which combines high-frequency waves and laser to relieve pain by providing in-depth heat, and is used by dentists.
Q. What are your future plans and blueprint after entering the global market?
Aiming to become a globally renowned company by expanding the sales in the U.S. market
A. With a network that expands over 50 countries around the world, including the United States and Europe, 75% of our sales comes from exports. In terms of our sales, 40% is from the U.S. market, and 30% is from the European market. We plan to continuously expand our sales to advanced countries, while also increasing our sales in emerging countries in the Middle East and Asia. Reepot, our next-generation laser device, was recently approved by the U.S. FDA. We will aim to get Reepot approved by other countries including European countries and actively expand sales in the U.S. market to secure a growth momentum for our company. By focusing on the three principles of success for aesthetic devices, which are sales in consumables, export, and securing our own brand, ILOODA will continue to grow as a company.